All of you will be able to count to ten in the Alkane family
You must learn what you're dealing with......
C1 Methane C6 Hexane
C2 Ethane C7 Heptane
C3 Propane C8 Octane
C4 Butane C9 Nonane
C5 Pentane C10 Decane
A little chemistry will go a long ways in understanding how and why things happen in the analyzer world.
Elements ------- Compounds --------- Mixtures
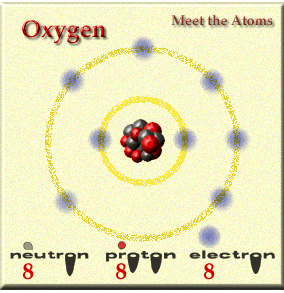
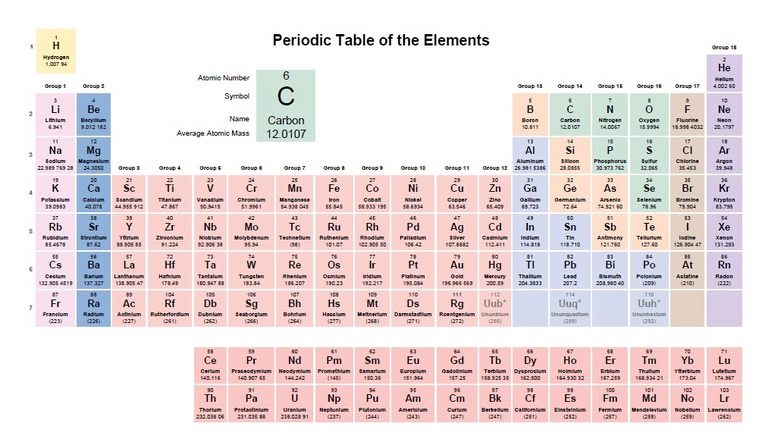
Elements are the simplest substance that can exist. Our has around 100 elements and a few of them are hydrogen, carbon, and sulfur. ( Students, print out the periodic table )
Compounds are made when we combine elements and they can only be changed chemical or electrical means. Such as H2O --- water.
Mixtures are when two or more materials can be mixed together and then seperated into the original means like oil.
Elements are made up of atoms. Not all atoms are the same and they vary in weight and structure.
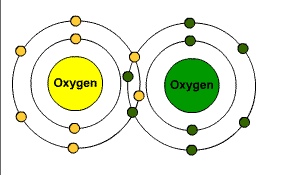
Elements are made up of atoms and you combine elements to form compounds and the atoms change when doing this and it becomes a molecule. What is a molcule of oxygen? O2..... two individual O join and become one.
As we have discussed in class. A lot of the material you need to learn revolves around Carbon. So get to know it. It's Atomic number is 6. It's Symbol is C. It's Atomic Weight is 12.01 and most important is that it has four bonds and they will make a double bond if they have to but don't like it. What is a triple bond Hydrocarbon?
Hint.....
It's really mad and ready to explode.
As an analyzer technician you will need to have a grasp of the periodic table. You will have the atomic number and that is just where it comes in on the table. There will be a symbol of the element and the bottom number is what you need to concentrate on - atomic weight. The table is based on C (Carbon) = 12. I have pulled out some different components and shown their Atomic Weight/Mass.
Atomic Mass
H2O Water = 18 He Helium = 4
CO Carbon Monoxide = 28 C2H6 Ethane = 30
N2 Nitrogen = 28 C1H4Methane = 16
O2 Oxygen = 32 C3H8 Propane = 44
CO2 Carbon Dioxide = 44 C5H12 Pentane = 72
Cl2 Chlorine = 70 H2S Hydrogen Sulfide = 34
C3H4 Propadiene = 40
When a component is looking to fill its outer shell with an electron because it has a valence there is a couple of ways this can happen. It can either share an electron or steal it.
Covalent Bond is when they share.
Ionic Bond is when it steals.